TECO Aspergillus Galactomannan Assay
Key Features
Detection of Aspergillus galactomannan antigen for the diagnosis of Invasive Aspergillosis (IA)

Product Description
The mortality rate of Invasive Aspergillosis is between 60 and 100% without timely treatment, thus reliable determination of galactomannan antigen may be essential.
- Sandwich ELISA as diagnostic gold standard
- Validated for serum and BAL samples
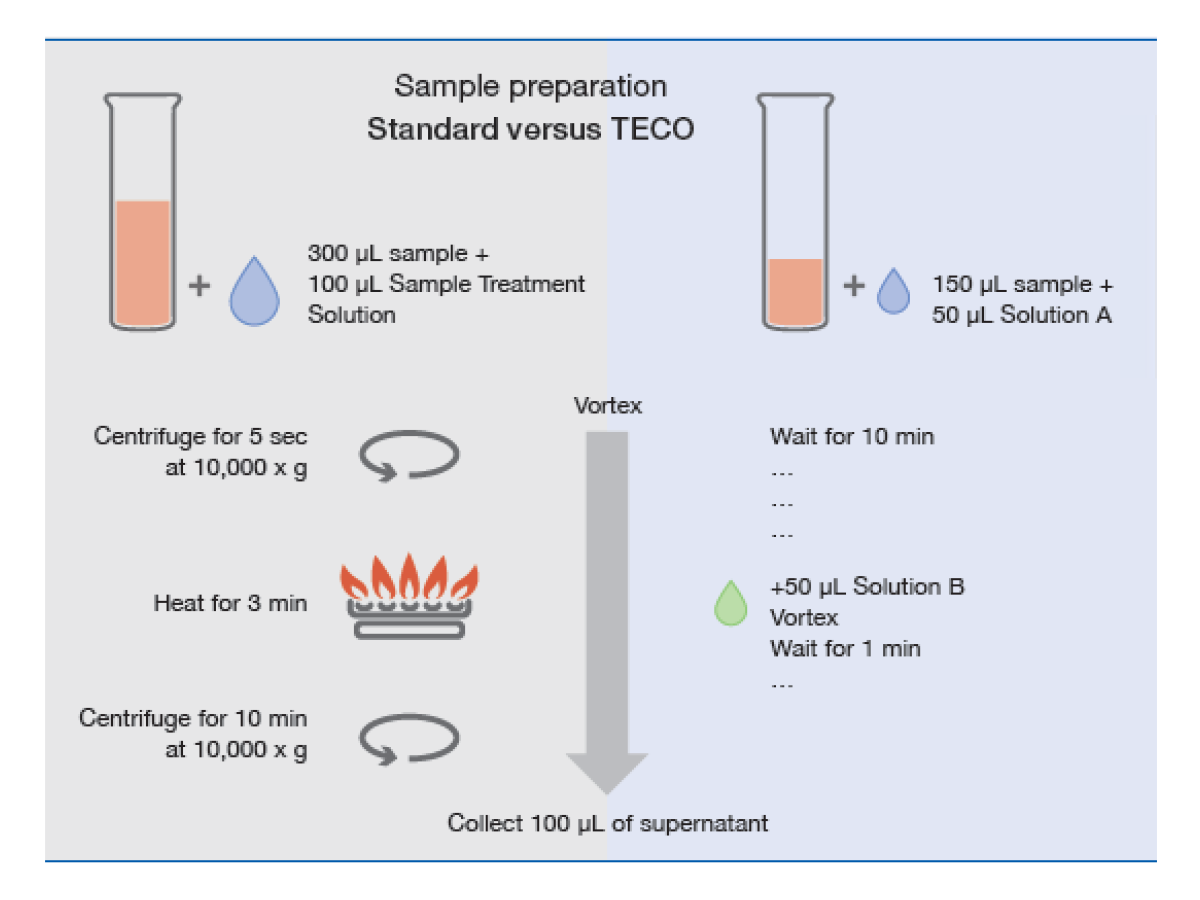
- Significantly simplified sample preparation
- Only 150 µL sample volume required
- Limit of detection: 0.67 ng/ml (serum); 1.09 ng/ml (BAL)
- Automation possible from sample to final result
Detection of galactomannan antigens as indicators of Aspergillus spec. infection using anti-galactomannan monoclonal antibodies and HRP-mediated color reaction. Established diagnostic procedure, significantly simplified sample preparation.
Request Quotation
Request Documentation
Associated Products
| Product | |||
|---|---|---|---|

|
TECO Fungus (1-3)-B-D-Glucan AssaySKU : TE1068 |
TECOmedical | View |





